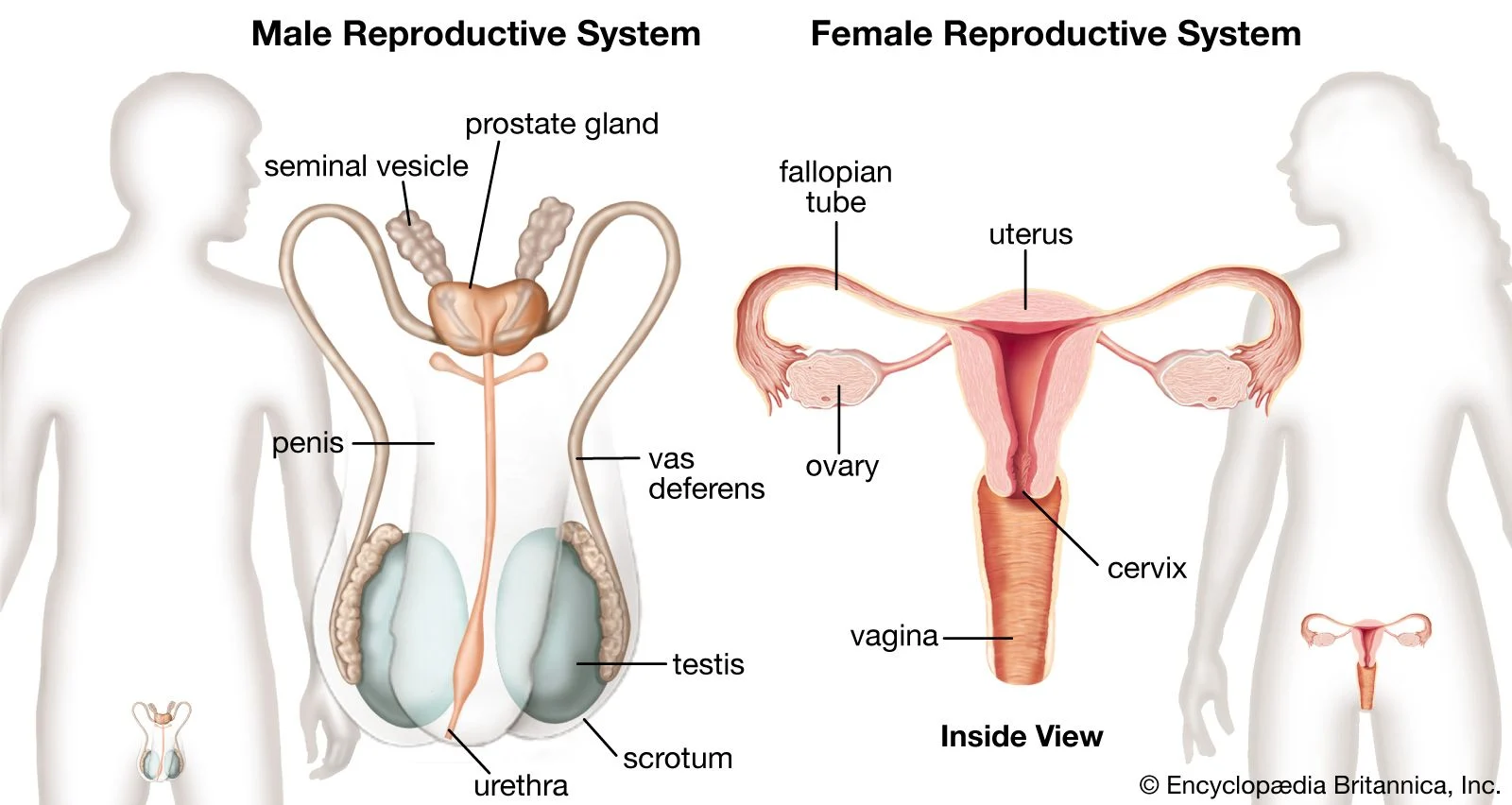
 Can you do self-insemination at home ?
Can you do self-insemination at home ?
Pharmaceutical giant Pfizer has requested the Food and Drug Administration (FDA) to extend the emergency use authorization of its Covid-19 vaccine to include children and teenagers aged 12 to 15. This move follows promising results from clinical trials indicating that the vaccine is both safe and 100 percent effective in preventing Covid-19 in this age group. The FDA had initially granted emergency use approval for individuals aged 16 and older late last year.
This request comes at a time when rising Covid-19 cases among younger populations are contributing to outbreaks in several states, according to NBC News. Pfizer announced that the trials showed similar side effects in teens to those experienced by adults, which include sore arms, fatigue, and headaches. “We are hopeful to initiate vaccinations for this age group before the upcoming school year,” said Pfizer CEO Mark Thomas in a statement regarding the findings.
Expanding Research to Younger Children
In addition to focusing on the 12 to 15 age bracket, Pfizer is also conducting research on the vaccine’s effectiveness in children aged 6 months to 11 years, with the first doses in that trial administered in March. “We recognize the importance of expanding vaccine authorization to younger populations and are optimistic about the data from adolescents,” Thomas commented.
Furthermore, participants in the trial who received the vaccine exhibited levels of neutralizing antibodies comparable to those found in older vaccinated teens and young adults. Pfizer’s Phase 3 trials in adults indicated that the vaccine was approximately 95 percent effective in preventing symptomatic Covid-19, though real-world data from the Centers for Disease Control and Prevention showed a slight decrease to around 90 percent.
The Importance of Vaccinating Teenagers
Dr. Emily Johnson, a pediatric infectious disease specialist at a leading medical center, emphasized that vaccinating teenagers is crucial for navigating the pandemic. “By broadening the age range eligible for the vaccine, we can better protect vulnerable populations, including older adults and those with underlying health conditions,” she stated.
As the U.S. continues its push to vaccinate adults, the inclusion of children in vaccination efforts represents a significant step toward achieving herd immunity, and any advancements in this area are encouraging.
Additional Resources
For additional insights, check out this other blog post on home insemination strategies. Also, for more information on fertility topics, visit this excellent resource on pregnancy and home insemination.
Search Queries:
Summary: Pfizer has requested FDA approval to extend its Covid-19 vaccine to children aged 12-15, highlighting safety and effectiveness in clinical trials. This initiative aims to address increasing Covid-19 cases among younger populations and is an important step toward achieving herd immunity.