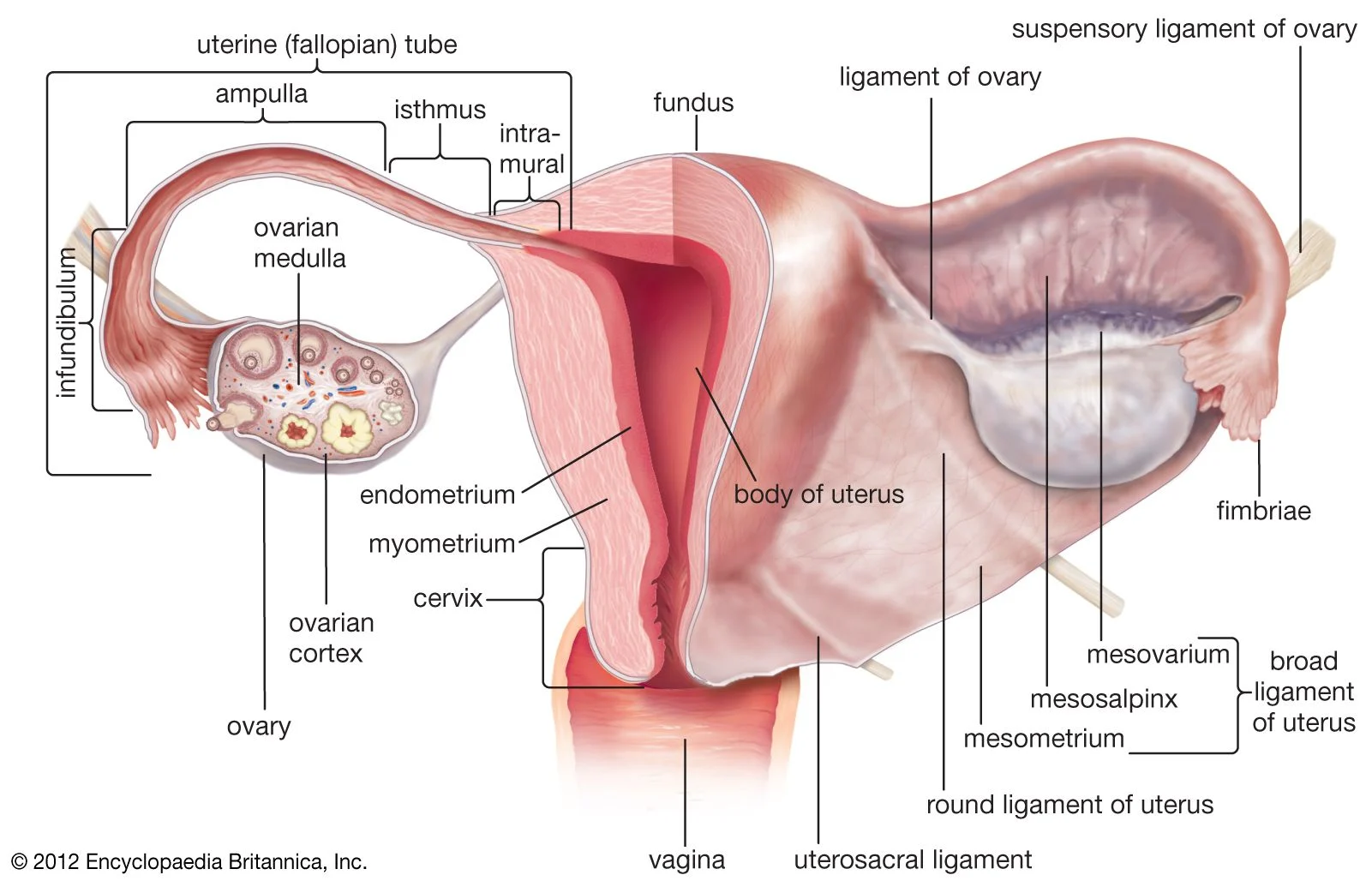
 Can you do self-insemination at home ?
Can you do self-insemination at home ?
The FDA is on the verge of granting emergency use authorization for Pfizer’s COVID-19 vaccine for children aged 12 and older, with an announcement expected as soon as next week. Vaccination rates among adults in the U.S. have begun to plateau, indicating that many adults who are interested in receiving the vaccine have already done so. Currently, all adults are eligible for vaccination, with data showing that around 50% have received at least one dose and nearly a third are fully vaccinated. The next significant step is to secure authorization for vaccination in children, especially with the upcoming school year approaching.
In March, Pfizer submitted data from a clinical trial involving nearly 2,300 adolescents between the ages of 12 and 15. In this trial, half of the participants received the two-dose vaccine regimen that has already been administered to adults, while the other half received a placebo. Pfizer reported that the vaccine elicited a stronger immune response in adolescents compared to younger adults, with only 18 cases of COVID-19 observed in the trial, all among those who received the placebo. These findings suggest that the vaccine offers similar protection to children as it does to adults.
This development is promising, especially considering that while children are less likely to experience severe COVID-19 symptoms, approximately 300 children in the U.S. have died from the virus since the start of the pandemic, which has claimed nearly 600,000 lives overall. If the FDA grants the emergency use authorization, the next step will involve a CDC panel that will establish guidelines on distribution and determine if certain groups, such as children with underlying health conditions, should be prioritized.
Meanwhile, Moderna, another vaccine manufacturer, is conducting clinical trials for its vaccine in teenagers and younger children, with results anticipated later this summer. For more information on home insemination and related topics, you can check out this blog post, which discusses various aspects of pregnancy.
If you’re curious about home insemination methods, there’s a wealth of information available. For a deeper dive, visit this authoritative site, or explore this excellent resource on pregnancy and home insemination.
Search Queries:
- Home insemination techniques
- Self insemination methods
- Home insemination kit reviews
- Pregnancy options for singles
- Conceiving at home
In summary, the FDA is preparing to authorize Pfizer’s COVID-19 vaccine for children aged 12-15, which marks an important step in the ongoing vaccination efforts as schools prepare to reopen. Following FDA approval, the CDC will set guidelines for distribution, particularly regarding vulnerable groups.