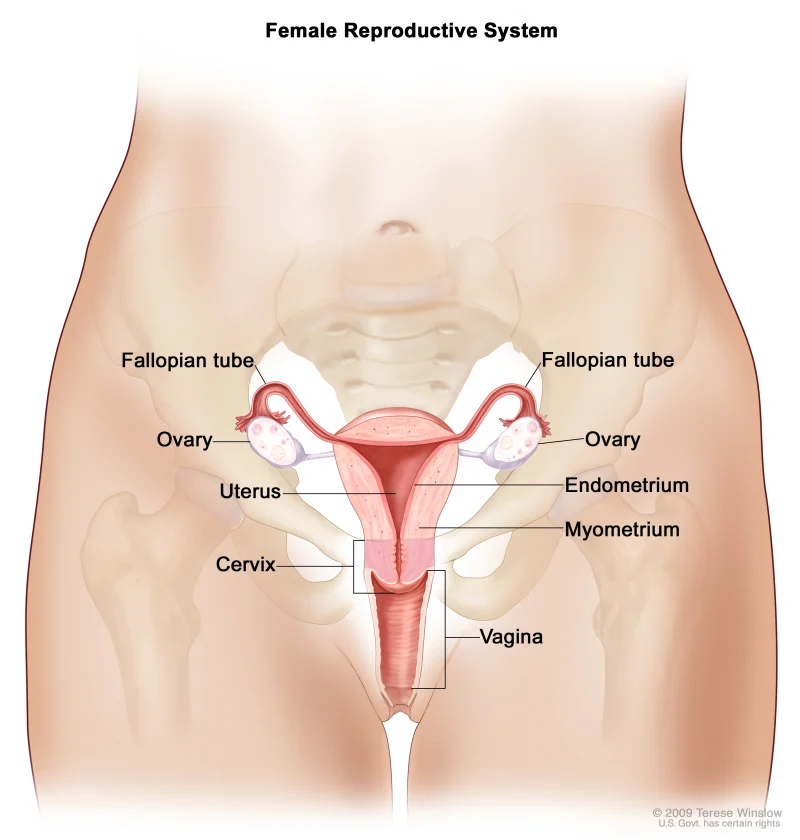
 Can you do self-insemination at home ?
Can you do self-insemination at home ?
The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) have announced a temporary suspension of the Johnson & Johnson vaccine at federal vaccination sites. This decision follows reports of rare blood clotting disorders in six individuals who received the vaccine, all of whom were adult women aged 18 to 48. According to the New York Times, this pause aims to allow states to assess the vaccine’s safety for continued use.
While the news may sound concerning, it’s important to note that nearly 7 million doses of the Johnson & Johnson vaccine have been administered in the U.S., with the occurrence of adverse events being extremely rare. For perspective, hormonal birth control can lead to blood clots in approximately 3 to 9 women out of every 10,000 annually. Most hormonal contraceptives are considered safe and effective for the majority of healthy individuals.
Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, stated that the pause is a precautionary measure. The six reported cases involved blood clots in conjunction with low platelet counts, and one individual has tragically died while another is critically ill. These reactions occurred between 6 and 13 days post-vaccination.
Both agencies are actively investigating the potential links between the vaccine and this rare blood clotting disorder. The CDC is convening the Advisory Committee on Immunization Practices (ACIP) to review these cases and their implications thoroughly. The pause is also intended to ensure that healthcare providers are aware of these adverse events and can manage them appropriately, given the specific treatment needed for such blood clots.
Johnson & Johnson has expressed its commitment to working closely with health authorities and medical experts during this time. Many states, including Ohio, New York, and Connecticut, are already halting the use of this vaccine as further investigations unfold.
Recent recipients of the Johnson & Johnson vaccine are advised to consult their healthcare providers if they experience severe headaches, abdominal pain, leg pain, or shortness of breath within three weeks of receiving the vaccine. However, experts emphasize that the risk of such adverse effects remains exceedingly low. “You’re talking about 1 per million,” noted Dr. Carlos del Rio from Emory University, highlighting the rarity of these events compared to the millions of doses administered.
For more insights on this topic, you can explore additional resources like this informative article here and here. For those interested in pregnancy and home insemination, this site offers excellent information.
Summary
The FDA and CDC have temporarily paused the Johnson & Johnson vaccine due to rare blood clotting cases among recipients. This measure is precautionary, and while concerning, adverse events remain extremely rare among the millions of doses given. States are advised to assess the situation as health authorities investigate further.
SEO Metadata
FDA, CDC, Johnson & Johnson vaccine, blood clotting, vaccine safety, health news