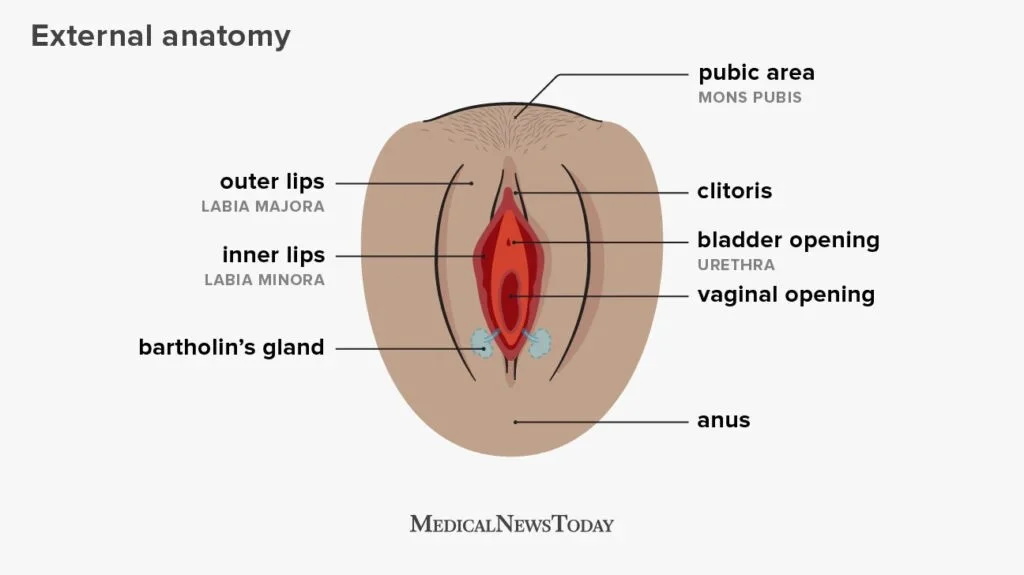
 Can you do self-insemination at home ?
Can you do self-insemination at home ?
A recent study reveals that pregnant and breastfeeding women who receive the COVID-19 vaccine generate a strong immune response, comparable to that of women who are neither pregnant nor breastfeeding. This finding is crucial for expectant mothers weighing their vaccination options, especially since COVID-19 poses a higher risk of severe illness, hospitalization, and death in pregnant individuals.
The research, published in the American Journal of Obstetrics and Gynecology, indicates that antibodies produced by vaccinated mothers are present in both umbilical cord blood and breast milk, suggesting the possibility of transferring immunity to their infants. Dr. Liam Carter, a maternal-fetal medicine expert at a major Boston hospital and co-author of the study, expressed that this development provides valuable data for obstetricians and healthcare providers who previously faced a significant lack of information due to the exclusion of pregnant and breastfeeding women from initial vaccine trials.
The study involved 131 women of reproductive age, monitored at two medical facilities: 84 were pregnant, 31 were breastfeeding, and 16 served as a control group. All participants received both doses of either the Pfizer or Moderna vaccines. Analysis of their blood revealed that the antibody levels induced by the vaccine were consistent across all groups. Furthermore, the antibodies were found in umbilical cord blood and breast milk, reinforcing the idea that vaccinated mothers may provide some level of protection to their babies, although the duration of this immunity remains uncertain.
Interestingly, the antibody levels generated by the vaccine were significantly higher than those found in women who had previously contracted COVID-19. While the study did not specifically evaluate the overall safety of the vaccine for pregnant women, existing evidence suggests that the vaccines are unlikely to pose significant risks to expectant mothers, as highlighted by the Centers for Disease Control and Prevention (CDC). Animal studies have shown no safety concerns, and while the mRNA technology used in Pfizer and Moderna vaccines is relatively new, the Johnson & Johnson vaccine has previously been administered to pregnant women during an Ebola vaccination campaign without adverse effects.
This study focused on vaccinations during the third trimester, with further research ongoing to determine the optimal timing for vaccination. Dr. Carter advises that eligible women should receive the vaccine as soon as possible. The American College of Obstetricians and Gynecologists supports the vaccination of pregnant and breastfeeding women, emphasizing that such individuals should have the autonomy to decide on vaccination, given the heightened risks associated with COVID-19 during pregnancy.
For those seeking more information about pregnancy and related topics, check out this excellent resource and learn about home insemination at Home Insemination Kit. For additional insights, visit Intracervical Insemination.
Search queries you might find interesting:
- COVID-19 vaccine for pregnant women
- Breastfeeding and vaccine antibodies
- Pregnancy and COVID-19 risks
- Expecting mothers and vaccination
- Vaccine safety in pregnancy
In summary, the study highlights that pregnant and breastfeeding women demonstrate a strong immune response to the COVID-19 vaccine, providing hope for maternal and infant health. The presence of antibodies in umbilical cord blood and breast milk indicates potential immunity transfer to babies, reinforcing the importance of vaccination in this demographic.